CHECKPOINT INHIBITORS
A recent breakthrough cancer study reported in The New England Journal of Medicine showed that treatment with the drug dostarlimab resulted in complete remissions of early-stage rectal cancers in every treated patient.
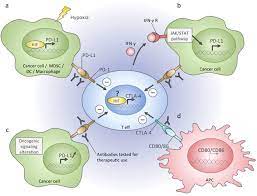
Approved by the FDA in August last year, dostarlimab is a kind of cancer immunotherapy treatment referred to as a “checkpoint inhibitor.” The name comes from the fact that checkpoint inhibitors block (i.e., inhibit) the brakes (i.e., checkpoints) that tumors use to fend off the immune system’s T cells.
The first significant implication of this study is that checkpoint inhibitors can be applied to early-stage rectal cancers with incredible efficacy. Conceivably, this could eliminate the need for surgery, radiation, and chemotherapy one day—with immune memory preventing future cancer spread.
Additionally, the current rectal cancer standard of care requires surgery. If the cancer is too close to the anus, patients need AP resections that require a colostomy bag for waste elimination. With checkpoint inhibition therapy, rectal cancer patients may now avoid this and drastically improve their quality of life.
1. #NFT
2. #AFL
3. #ForeignPolicy
4. #MonkeyPox
5. #NewPM
6. #Ukraine
7. #Australian Cricket
8. #SaudiGolfTour
9. #PlatinumAnniversary
10. #BorisHair

No comments:
Post a Comment